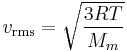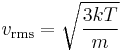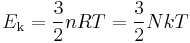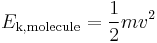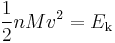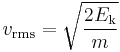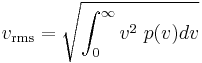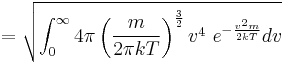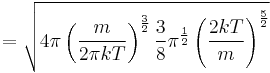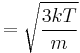Root-mean-square speed
Root-mean-square speed is the measure of the speed of particles in a gas that is most convenient for problem solving within the kinetic theory of gases. It is defined as the square root of the average velocity-squared of the molecules in a gas. It is given by the formula[1]
where vrms is the root mean square of the speed, Mm is the molar mass of the gas, R is the molar gas constant, and T is the temperature in kelvin. Although the molecules in a sample of gas have an average kinetic energy (and therefore an average speed) the individual molecules move at various speeds, i.e. they exhibit a distribution of speeds. Some move fast, others relatively slowly. Collisions change individual molecular speeds but the distribution of speeds remains the same. This equation is derived from kinetic theory of gases using Maxwell–Boltzmann distribution function. The higher the temperature, the greater the mean velocity will be. This works well for both nearly ideal, atomic gases like helium and for molecular gases like diatomic oxygen. This is because despite the larger internal energy in many molecules (compared to that for an atom), 3RT/2 is still the mean translational kinetic energy. This can also be written in terms of the Boltzmann constant (k) as
where m is the mass of one molecule of the gas.
This can be derived with energy methods:
where Ek is the kinetic energy and N is the number of gas molecules.
Given that v2 ignores direction, it is logical to assume that the formula can be extended to the entire sample, replacing m with the entire sample's mass, equal to the molar mass times the number of moles n yielding
Therefore
which is equivalent.
The same result is obtained by solving the Gaussian integral containing the Maxwell speed distribution, p(v):
References
- ^ Raymond A. Serway, Jerry S. Faughn, and Chris Vuille (2011). College Physics, Volume 1 (9th ed.). 9780840068484. p. 352. http://books.google.com/books?id=HLxV-IKYO5IC&pg=PA352.